Publication Highlight
Digital Melting Curve Analysis for Multiplex Quantification of Nucleic Acids on Droplet Digital PCR (1)
The Challenge in Digital PCR Multiplexing
Conventional droplet digital PCR (ddPCR) relies on fluorescence channels to differentiate between targets. For example: (2). For example:
- FAM channel = Target A
- HEX channel = Target B
- Cy5 channel = Target C
This works well for 2–4 targets, but once the available spectral bandwidth is exhausted, adding more targets means adding more dyes (increasing cost and complexity) or multiple reactions (increasing turnaround time).
The bottleneck: The number of targets is traditionally limited by the number of optical channels in the instrument.
The Innovation: Digital Melting Curve Analysis
The featured study overcame this limitation by combining ddPCR with melting temperature (Tm) profiling (3).
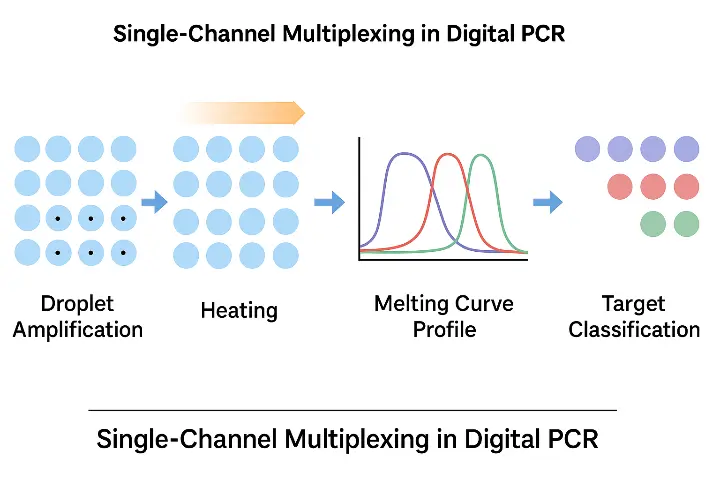
Step-by-step workflow:
-
Single dye for all targets
All probes or intercalating dyes are detected in the same fluorescence channel. - Amplification in droplets – Standard ddPCR partitioning ensures each droplet contains zero or one target molecule.
- Incremental heating – After amplification, droplets are gradually heated.
- Droplet-by-droplet melting analysis – Each amplicon’s unique Tm (determined by GC content and length) creates a distinctive melting profile.
-
Classification by melting peaks – Software assigns each droplet to its target based on peak temperature.
Digital MCA vs. Conventional qPCR MCA
Unlike conventional qPCR melting curve analysis, which measures the bulk signal of the entire reaction well, digital MCA captures melting data from individual droplets (4)(5).
|
Feature |
qPCR MCA |
Digital MCA |
|---|---|---|
|
Resolution |
Peaks may overlap |
Individual droplet peaks resolved |
|
Multiplex limit |
1–2 targets/dye |
5–6 targets/dye |
|
Sensitivity |
Limited by background noise |
High — droplets act as isolated microreactors |
4 | Sniper Digital PCR enables This Method
The Sniper DQ24 ddPCR system is uniquely suited for digital MCA because it:
- Produces highly uniform droplets for consistent Tm measurement.
- Offers real-time imaging across the droplet field for accurate melting profile capture.
- Provides precise thermal ramp control post-amplification.
- Integrates image analysis software to automatically classify droplets based on melting peaks (6).
In the study, this enabled detection of six respiratory pathogens in a single fluorescence channel with ~85% classification accuracy.
Advantages for Research and Diagnostics
- Cost savings – No need for additional dyes or channels.
- High multiplexing capacity – Up to 6 targets per channel.
- Rapid adaptation – Existing single-channel assays can be multiplexed.
-
Field-deployable potential – More targets, same instrument footprint.
6 | References
- Fang, X., Zhang, Y., et al. (2025). Digital Melting Curve Analysis for Multiplex Quantification of Nucleic Acids on Droplet Digital PCR. Biosensors, 15(1), 36.
- Huggett, J. F., Whale, A. S. (2013). Digital PCR as a novel technology and its potential implications for molecular diagnostics. Clinical Chemistry, 59(12), 1691–1693.
- Vossen, R. H. A. M., et al. (2009). High-Resolution Melting Analysis (HRMA) — More than just sequence variant screening. Human Mutation, 30(6), 860–866.
- Hindson, C. M., et al. (2013). Absolute quantification by droplet digital PCR versus analog real-time PCR. Nature Methods, 10, 1003–1005.
- Whale, A. S., et al. (2016). Fundamentals of multiplexing with digital PCR. Biomolecular Detection and Quantification, 10, 15–23.
-
Sniper Medical Technology Co., Ltd. (2024). Sniper DQ24 Droplet Digital PCR System – Technical Overview.
7 | Learn More
Read the full study here: MDPI – Digital Melting Curve Analysis for Multiplex Quantification of Nucleic Acids on Droplet Digital PCR
Explore the Sniper DQ24 Digital PCR platform here: Sniper Digital PCR @ Darwin Science
#DigitalPCR #MeltingCurve #SingleChannelMultiplexing #ddPCR #MolecularDiagnostics #SniperPCR #MultiplexAssay