Why rotavirus still needs better molecular tools
Rotavirus remains a leading cause of severe paediatric gastro-enteritis, responsible for ~200 000 deaths in children < 5 years annually. Two genotypes that increasingly break through vaccine protection—G3P[8] and G9P[8]—demand sensitive, standardised detection for clinical diagnosis, vaccine-effectiveness studies and environmental monitoring. Traditional RT-qPCR struggles at the low viral loads seen post-vaccination and offers no mechanism to create metrologically traceable reference standards. Digital PCR (dPCR) solves both problems by partitioning reactions into thousands of nanolitre droplets so every target molecule is individually amplified and counted, delivering absolute copy numbers without external calibrators .
What the 2025 rotavirus paper demonstrates
Yang et al. developed the first SI-traceable whole-virus reference materials for the G3P[8] and G9P[8] strains and an accompanying one-step RT-dPCR method. Five commercial dPCR instruments were benchmarked; the Sniper DQ24 Plus was among the three droplet systems selected for the multi-laboratory validation. Key outcomes:

One competing platform underestimated copy number by ~30 %, but the DQ24 Plus stayed well inside the ±15 % accuracy window, underscoring its suitability for regulated viral-load testing.
How the Sniper DQ24 Plus a dds value
- Traceable accuracy out-of-the-box — The DQ24 Plus matched the Bio-Rad QX200® reference values in the study, meaning laboratories can report rotavirus copy numbers with confidence on the first run.
- Walk-away ease — A 24-well chip with on-board droplet generation reduces hands-on time; six optical channels leave head-room for duplex or multiplex VP4/VP7 typing without hardware changes.
- Consistent droplet volume (~0.8 nL) — Identical to the reference procedure, so no scaling factors or empirical corrections are needed when adopting the published method.
- Fast turnaround — Complete RT-dPCR run in ≈ 2 hours lets surveillance labs deliver same-day results for outbreak response.
Wider impact of dPCR on virus research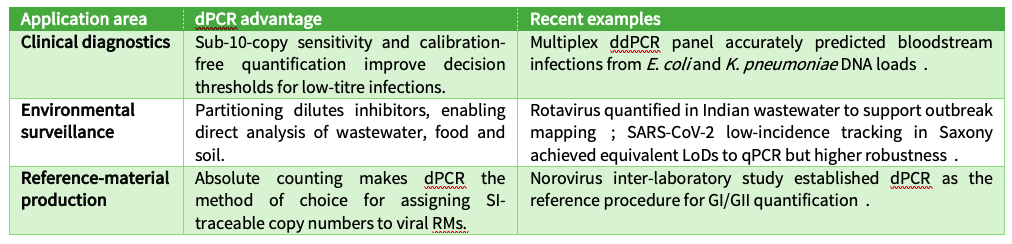
Take-home message for laboratories and decision-makers
Digital PCR is no longer an experimental add-on: it is a mature, regulator-endorsed technology delivering the sensitivity, accuracy and traceability modern virology requires. The 2025 rotavirus study shows that the Sniper DQ24 Plus™ not only meets but exceeds the analytical standards demanded for global viral-surveillance programmes. Adopting DQ24 Plus lets your lab:
- Align with new rotavirus reference materials immediately for harmonised reporting across studies and borders.
- Future-proof multiplex capability as new genotypes emerge or when simultaneous vaccine-strain monitoring is needed.
- Differentiate your service offering with traceable results that clinicians, public-health agencies and peer reviewers can trust.
For protocol details, instrument demonstrations or pricing, contact Darwin Science — your authorised Sniper DQ24 Plus distributor.
References
- Yang J, Zhang Y, Liu M, et al. Development of RT-dPCR method and reference material for rotavirus G3P[8] and G9P[8]. Anal Bioanal Chem. 2025;417:2513-2523. doi:10.1007/s00216-024-05690-2. 16-024-05690-2 (1).pdf](file-service://file-DQDARhcLSiqcQTSVSgVzFg)
- Domínguez L S, Suárez A C, Sánchez M, Ledesma J. Advancing pathogen identification: the role of digital PCR in infectious-disease diagnostics and environmental surveillance. Diagnostics. 2024;14(15):1598.
- Nema R K, Singh A K, Nagar J, et al. Investigating the presence of rotavirus in wastewater samples of Bhopal region, India, by utilizing droplet digital PCR. Cureus. 2024;16(4):e58882. doi:10.7759/cureus.58882.
- de la Cruz Barron M, Kneis D, Geissler M, et al. Evaluating the sensitivity of droplet digital PCR for the quantification of SARS-CoV-2 in wastewater. Front Public Health. 2023;11:1271594.
- International inter-laboratory study comparing dPCR for norovirus GI/GII RNA quantification. J Virol Methods. 2024; 329:114818.