1 | Digital PCR: a new gold standard for residual-disease testing
Digital PCR (dPCR) partitions each reaction into thousands of nanolitre droplets so that individual target molecules are amplified and counted. Unlike RT-qPCR, dPCR delivers absolute copy numbers without external calibrators, giving up to ten-fold better sensitivity at low transcript levels and <5 % imprecision at routine clinical loads . For chronic myeloid leukaemia (CML), where treatment decisions hinge on detecting BCR-ABL1 transcripts at MR4–4.5, this extra accuracy can determine whether a patient continues or discontinues TKI therapy.
2 | What the latest multi-centre study shows
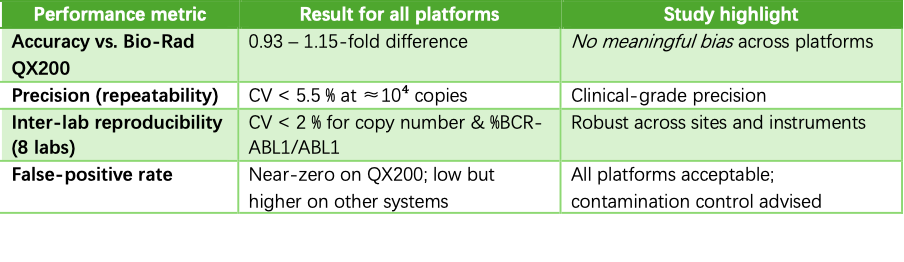
A 2024 reference-measurement study established the first SI-traceable RNA material for BCR-ABL1 P210 and compared five commercial dPCR platforms against the new standard . Key findings:
3 | Sniper DQ24 Plus — validated, reliable and ready for the clinic
The Sniper DQ24 droplet dPCR was one of the three instruments selected for the inter-laboratory arm of the study, alongside the market-dominant QX200 and the Microdrop-100 . Its performance underscores three advantages:
- Traceable accuracy – DQ24 copy-number results fell well within the ±15 % acceptance window set by the reference method, confirming that laboratories can report BCR-ABL1/ABL1 on the International Scale with confidence.
- Tight precision across sites – When eight independent laboratories quantified the new RNA reference material, DQ24 data contributed to an overall CV < 2 %, meeting ISO 5725 reproducibility criteria . That level of consistency translates into fewer repeat assays and clearer clinical cut-offs.
- Clinical-sample resilience – The platform’s accuracy was unaffected by high wild-type ABL1 background, mirroring real patient specimens where leukocyte RNA is abundant .
Additional DQ24 features your lab will appreciate
- 24-well chip format with on-board droplet generation for walk-away convenience.
- Partition volume ≈ 0.8 nL, aligning with the droplet size used for the reference procedure, so no scaling factors are needed.
- Intuitive Sniper-Quant software delivers ready-to-report copy numbers and %IS in a single click.
4 | Take-home message for labs and clinicians
- Digital PCR is ready for routine CML monitoring, delivering absolute quantification where RT-qPCR plateaus.
- The Sniper DQ24 Plus matched—or bettered—established systems for accuracy and precision in a peer-reviewed, SI-traceable study.
- Adopting DQ24 now lets your laboratory align with the newest international reference materials, future-proofing your BCR-ABL1 reporting and giving clinicians the confidence to manage therapy discontinuation safely.
References
- Qian Y. et al. Establishment of genomic RNA reference materials for BCR-ABL1 P210 measurement. Analytical & Bioanalytical Chemistry. 2024; DOI: 10.1007/s00216-024-05492-6.
- Huggett J.F., Whale A.S. Digital PCR as a transformative technology in the quantitation of nucleic acids. Clinical Chemistry. 2013;59(12):1670-1682.
- Basu A.S. Digital assays Part I: partitioning statistics and digital PCR. SLAS Technology. 2017;22(4):369-386.